Abstract
Background: Central nervous system relapses (CNSR) are uncommon in primary mediastinal large B-cell lymphoma (PMLBCL) with a reported incidence of 2.3-3.8% in the Rituximab era. Clinical risk factors for CNSR have been recognized in diffuse large B-cell lymphoma (DLBCL), but may not be applicable in PMLBCL, a pathobiologically distinct entity with constitutive activation of NFκB and JAK-STAT pathways and Programmed Death (PD) Ligand 1 and 2 overexpression.
Aim: To evaluate the incidence of first CNSR events without prior systemic progression and explore prognostic associations with baseline characteristics, CNS-IPI, and induction immunochemotherapy.
Methods: This is a retrospective study of a multinational cohort of 596 adult PMLBCL patients treated with R-CHOP or DA-EPOCH-R immunochemotherapy with or without radiotherapy between 2000 and early 2022. The cumulative incidence of first CNSR (CI-CNSR) was estimated considering the competing risks of death from any cause or prior systemic disease relapse/progression.
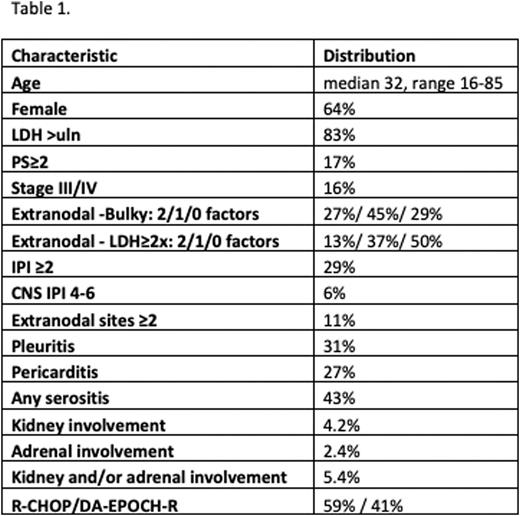
Results: Baseline characteristics are shown in Table 1. Only 17 patients (2.9%) received CNS prophylaxis [high-dose methotrexate (HD-MTX) 9, intrathecal MTX±other 7, both 1]. Kidney and/ or adrenal involvement at diagnosis was highly correlated with advanced stage, ≥2extranodal sites and high CNS-IPI (4-6) (p<0.001), with the vast majority of the latter also having kidney and/or adrenal involvement. With a 55-month median follow-up [interquartile range (IQR) 32-97 months], all 10 first CNSR events(9 isolated and 1 associated with systemic relapse) were recorded within 2 years of diagnosis [median time7.5 months, IQR 6-8, range 5-13 months], for a 2-year CI-CNSR of 1.78% (95% CI 0.9-3.2%). Two of 10 CNSR cases had received CNS prophylaxis with HD-MTX. All CNSR were parenchymal and only 2/8 were successfully salvaged (2 still under treatment). Four patients with CNSR had kidney involvement (plus adrenal in 2/4). Two of seven patients with both kidney and adrenal involvement and 2/ 17 with kidney infiltration only relapsed in the CNS. Kidney [subhazard ratio (SHR) 15.4, p<0.001], adrenal (SHR 13.6, p=0.001), any kidney or adrenal involvement (SHR 12.5, p<0.001), and high CNS-IPI (4-6, SHR 13.0, p<0.001) were associated with CNSR in univariate analysis. All 4 patients with high CNS-IPI who experienced CNSR (out of 35), had also kidney and/or adrenal involvement. Looser but significant associations were observed with high IPI (2-5; SHR 6.3, p=0.009), advanced stage (III/IV; SHR 5.5, p=0.007), impaired performance status (≥2; SHR 6.6, p=0.005), and ≥2 extranodal sites (SHR 5.4, p=0.009) but not chemotherapy backbone (R-CHOP or DA-EPOCH-R), CNS prophylactic therapy, age, gender, B-symptoms, LDH elevations or other baseline labs, serositis or PMLBCL-specific prognostic indices (stage IV/ extranodal plus bulky or high LDH≥2x).
Conclusion: In PMLBCL, CNSR is rare and appears to be primarily associated with kidney and/or adrenal involvement. CNS-IPI is also strongly prognostic but highly correlated with kidney and/or adrenal involvement. No late (>2 years) occurrences were seen and 9/10 relapses were isolated in the CNS. No inferences regarding the value of primary CNS prophylaxis can be made. Even in this very large series multivariate is not reliable due to the small number of events. A larger multinational effort is warranted.
Disclosures
Gurion:Takeda: Honoraria; Medison Ltd: Honoraria; Gilead: Honoraria; Roche: Honoraria; Novartis: Honoraria. Katodritou:JANSSEN: Other: travel expenses, Research Funding; AMGEN: Honoraria, Research Funding; GSK: Honoraria, Other: travel expenses, Research Funding; Takeda: Honoraria, Other: research expenses, Research Funding; Sanofi: Research Funding; Integris Pharma: Honoraria; Karyopharm: Research Funding; Abbvie: Honoraria, Other: travel expenses, Research Funding. Tadmor:Janssen: Research Funding; AbbVie, Roche, Novartis, Sanofi, Takeda, Janssen, Pfizer: Consultancy, Honoraria, Speakers Bureau. Symeonidis:Servier SOBI: Membership on an entity's Board of Directors or advisory committees; Rafarm: Honoraria; Demo/Apopharma: Research Funding; WinMedica: Research Funding; Roche: Research Funding; Vianex: Research Funding; Abbvie, Amgen, Astra-Zeneca, BMS, GenesisPharma, Gilead, Glaxo, Integris, Janssen, Novartis, Pfizer, Sanofi, Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Research Funding. Terpos:Genesis: Honoraria, Research Funding; BMS: Honoraria; EUSA Pharma: Honoraria, Other: Travel expenses; Janssen: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; Novartis: Honoraria; Takeda: Honoraria, Other: Travel expenses, Research Funding; GSK: Honoraria, Research Funding; Amgen: Honoraria, Other: Travel expenses, Research Funding. Gafter-Gvili:Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Medison: Honoraria, Membership on an entity's Board of Directors or advisory committees. Panayiotidis:ABBVIE, NOVARTIS, GENESISPHARMA, BMS,Gilead, Glaxo, Integris, Janssen, Novartis, Pfizer: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal